Chemistry⁚ The Molecular Nature of Matter and Change
Chemistry⁚ The Molecular Nature of Matter and Change by Martin Silberberg and Patricia Amateis has been recognized in the general chemistry market as an unparalleled classic. The revision for the ninth edition focused on continued optimization of the text. To aid in this process, we were able to use data from literally thousands of student responses to questions in LearnSmart, the adaptive learning…
Introduction
Chemistry is a fundamental science that delves into the composition, structure, properties, and transformations of matter. It is a vast and intricate field that explores the building blocks of the universe, from the smallest atoms to the most complex molecules. The study of chemistry is essential for understanding the world around us, as it provides insights into the natural phenomena that shape our lives.
Chemistry plays a crucial role in numerous industries, including medicine, agriculture, manufacturing, and energy. It is the foundation for the development of new drugs, fertilizers, materials, and energy sources. Chemists are constantly working to solve critical challenges facing humanity, such as climate change, pollution, and disease.
In this comprehensive exploration of chemistry, we will embark on a journey to unravel the mysteries of the molecular nature of matter and change. We will delve into the fundamental principles of chemistry, from the basic building blocks of matter to the intricate reactions that govern chemical transformations.
The Nature of Matter
Matter is anything that occupies space and has mass. It exists in various forms, from the solid rocks beneath our feet to the air we breathe. The study of matter is central to chemistry, as it provides insights into the fundamental building blocks of the universe. Matter can be classified based on its composition and properties.
A pure substance is a form of matter with a fixed composition and distinct properties. Examples include elements like gold and oxygen, and compounds like water and salt. In contrast, a mixture is a combination of two or more substances that are not chemically bonded. Mixtures can be homogeneous, where the components are evenly distributed, or heterogeneous, where the components are unevenly distributed.
The states of matter, solid, liquid, and gas, are defined by the arrangement and movement of their particles. Solids have fixed shapes and volumes, liquids have fixed volumes but can change shape, and gases have no fixed shape or volume. These states of matter can be interconverted by changing temperature and pressure, illustrating the dynamic nature of matter.
Atoms and Elements
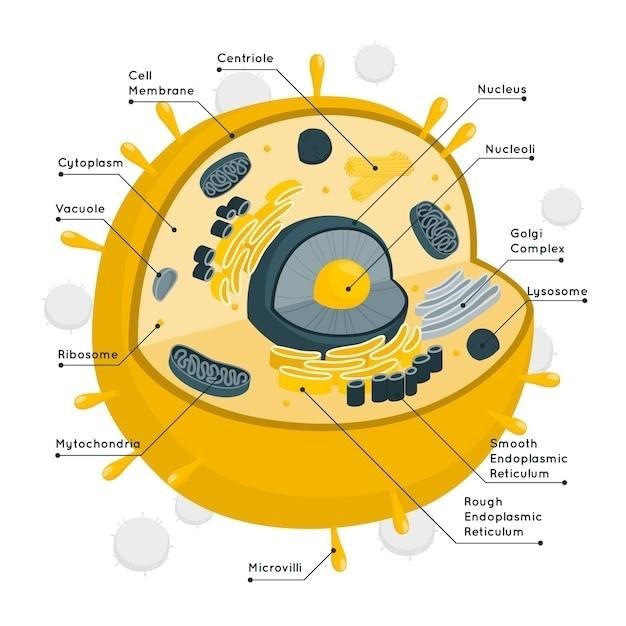
At the heart of matter lie atoms, the smallest units of an element that retain the chemical properties of that element. Elements are pure substances that cannot be broken down into simpler substances by ordinary chemical means. Each element is characterized by a unique atomic number, which represents the number of protons in the atom’s nucleus. These protons, along with neutrons, make up the atom’s nucleus, which is positively charged. Electrons, negatively charged particles, orbit the nucleus in specific energy levels or shells.
The concept of atoms dates back to ancient Greek philosophers, but it wasn’t until the early 19th century that John Dalton proposed his atomic theory, which laid the foundation for modern chemistry. Dalton’s theory stated that all matter is composed of atoms, that atoms of a given element are identical, and that chemical reactions involve the rearrangement of atoms. The discovery of the electron by J.J. Thomson and the subsequent development of the nuclear model by Ernest Rutherford further refined our understanding of the atom.
The periodic table, a systematic arrangement of elements based on their atomic numbers and electron configurations, is a powerful tool for understanding the relationships between elements and predicting their chemical behavior. It provides a framework for organizing the vast array of elements and their properties, making chemistry a more organized and predictable science.
Atomic Structure
The atom, the fundamental building block of matter, consists of a central nucleus surrounded by a cloud of negatively charged electrons. The nucleus, a tiny, dense region, contains positively charged protons and neutral neutrons. The number of protons, known as the atomic number, defines the element. For example, all carbon atoms have six protons, while all oxygen atoms have eight. The mass number, the total number of protons and neutrons, determines the atom’s mass. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons, resulting in different mass numbers.
Electrons occupy specific energy levels or shells around the nucleus, with each shell having a characteristic energy. The arrangement of electrons in these shells, known as the electron configuration, determines an atom’s chemical behavior. The outermost shell, known as the valence shell, contains valence electrons, which are involved in chemical bonding.
The understanding of atomic structure is crucial in explaining the chemical properties of elements. It helps us understand why different elements react differently, how atoms form molecules, and how chemical reactions occur. The atomic model has evolved over time, with advancements in technology leading to more sophisticated models that provide a deeper understanding of the atom’s structure and behavior.
The Periodic Table
The periodic table is a powerful tool that organizes the elements based on their atomic structure and recurring chemical properties. It is arranged in rows called periods and columns called groups. Elements within the same group share similar chemical properties due to having the same number of valence electrons. The periodic table is a visual representation of the periodic law, which states that the properties of elements are periodic functions of their atomic numbers.
The table is divided into metals, nonmetals, and metalloids. Metals are typically shiny, malleable, and good conductors of heat and electricity. Nonmetals are generally dull, brittle, and poor conductors. Metalloids exhibit properties of both metals and nonmetals. Each element is represented by a unique symbol, atomic number, and atomic mass. The periodic table provides a framework for understanding the relationships between elements and their chemical behavior, making it an essential tool for chemists and scientists.
The periodic table is a dynamic tool, constantly evolving as new elements are discovered and our understanding of atomic structure deepens. It serves as a foundation for numerous branches of chemistry, including inorganic chemistry, organic chemistry, and analytical chemistry. Its ability to predict and explain chemical behavior makes it an indispensable resource in the study of matter and its transformations.
Chemical Bonding
Chemical bonding is the force that holds atoms together to form molecules and compounds. It is a fundamental concept in chemistry, explaining the structure, properties, and reactivity of matter. There are two main types of chemical bonds⁚ ionic and covalent. Ionic bonds form when atoms transfer electrons, resulting in the formation of ions with opposite charges that attract each other. These bonds typically occur between metals and nonmetals.
Covalent bonds form when atoms share electrons, creating a stable arrangement where both atoms achieve a full outer shell of electrons. These bonds are typically found between nonmetals. The strength of a chemical bond depends on the type of bond, the number of electrons shared, and the distance between the atoms. Chemical bonds play a crucial role in determining the physical and chemical properties of substances.
Understanding chemical bonding is essential for comprehending chemical reactions, which involve the breaking and forming of bonds. It also provides insights into the structure and function of complex molecules, such as proteins, DNA, and pharmaceuticals. The study of chemical bonding continues to be a vital area of research, leading to advancements in materials science, nanotechnology, and medicine.
Chemical Reactions
Chemical reactions are processes that involve the rearrangement of atoms and molecules, leading to the formation of new substances with different properties. These reactions are governed by fundamental principles of chemistry, including the conservation of mass and energy. Chemical reactions can be represented by balanced chemical equations, which depict the reactants, products, and stoichiometry of the reaction.
There are various types of chemical reactions, categorized based on the changes they undergo. Some common types include synthesis reactions, where two or more reactants combine to form a single product; decomposition reactions, where a single reactant breaks down into two or more products; single displacement reactions, where an element replaces another element in a compound; and double displacement reactions, where two reactants exchange ions to form two new compounds.
Chemical reactions play a vital role in everyday life, from the combustion of fuels to the digestion of food. They are essential for industrial processes, such as the production of chemicals, materials, and energy. Understanding chemical reactions is crucial for developing new technologies and solving environmental challenges.
Stoichiometry
Stoichiometry is a fundamental concept in chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It allows us to predict the amount of reactants needed or products formed in a given reaction. The key principles of stoichiometry are based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction.
Stoichiometric calculations involve using balanced chemical equations and molar masses to determine the amounts of substances involved. The mole concept is central to stoichiometry, as it provides a standard unit for measuring the amount of substance. Molar mass, which is the mass of one mole of a substance, is used to convert between grams and moles.
Stoichiometry is essential for understanding chemical reactions and for carrying out laboratory experiments and industrial processes; It is used to determine the limiting reactant, which is the reactant that is completely consumed in a reaction and limits the amount of product formed. Stoichiometric calculations are also used to calculate theoretical yield, which is the maximum amount of product that can be obtained from a given amount of reactants.
Gases and the Kinetic-Molecular Theory
Gases are unique states of matter characterized by their ability to expand to fill any container and their compressibility. The behavior of gases is explained by the kinetic-molecular theory, which describes gases as consisting of tiny particles in constant, random motion. These particles are assumed to be point masses with negligible volume compared to the space between them.
The kinetic-molecular theory explains several key properties of gases⁚ pressure arises from the collisions of gas particles with the walls of their container; temperature is directly proportional to the average kinetic energy of the gas particles; diffusion and effusion are the result of the random motion of gas particles; and gas pressure is inversely proportional to volume (Boyle’s Law), directly proportional to temperature (Charles’s Law), and directly proportional to the number of moles of gas (Avogadro’s Law).
These laws are combined into the ideal gas law, which relates pressure, volume, temperature, and the number of moles of a gas. The ideal gas law provides a useful model for predicting the behavior of real gases under certain conditions. However, real gases deviate from ideal behavior at high pressures and low temperatures due to intermolecular interactions and the finite volume of gas particles.
Thermochemistry⁚ Energy Flow and Chemical Change
Thermochemistry is the study of heat flow and its relationship to chemical reactions. Heat is a form of energy that is transferred between objects at different temperatures. Chemical reactions can either release heat (exothermic reactions) or absorb heat (endothermic reactions). The amount of heat transferred in a chemical reaction is called the enthalpy change (ΔH), which is negative for exothermic reactions and positive for endothermic reactions.
The enthalpy change for a reaction can be calculated using Hess’s Law, which states that the enthalpy change for a reaction is independent of the pathway taken. Hess’s Law allows us to calculate the enthalpy change for a reaction even if it cannot be measured directly. Enthalpy changes can also be determined from standard enthalpy of formation values, which are the enthalpy changes associated with the formation of one mole of a compound from its elements in their standard states.
Thermochemistry is essential for understanding how energy is transferred and used in chemical reactions. It is also crucial in the design and optimization of chemical processes, such as the production of fuels and the development of new materials.
Solutions and Colligative Properties
A solution is a homogeneous mixture of two or more substances. The substance present in the larger amount is called the solvent, while the substance present in the smaller amount is called the solute. Solutions can be solid, liquid, or gaseous. The concentration of a solution refers to the amount of solute dissolved in a given amount of solvent.
Colligative properties are properties of solutions that depend on the concentration of the solute particles, but not on the identity of the solute. These properties include⁚
- Vapor pressure lowering⁚ The vapor pressure of a solvent is lowered when a nonvolatile solute is added.
- Boiling point elevation⁚ The boiling point of a solvent is raised when a nonvolatile solute is added.
- Freezing point depression⁚ The freezing point of a solvent is lowered when a nonvolatile solute is added.
- Osmotic pressure⁚ The osmotic pressure of a solution is the pressure that must be applied to prevent the flow of solvent across a semipermeable membrane.
Colligative properties are important in many applications, such as the determination of molecular weights, the preservation of food, and the design of desalination processes.
Equilibrium
Chemical equilibrium is a state where the rates of the forward and reverse reactions are equal, and the net change in concentration of reactants and products is zero. This does not mean that the reaction has stopped, but rather that the forward and reverse reactions are occurring at the same rate.
The equilibrium constant, K, is a value that describes the relative amounts of reactants and products at equilibrium. A large value of K indicates that the products are favored at equilibrium, while a small value of K indicates that the reactants are favored.
Le Chatelier’s principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress. These changes of condition can include changes in temperature, pressure, or concentration.
Equilibrium is a fundamental concept in chemistry and is used to understand and predict the behavior of chemical reactions. It is also important in many industrial processes, such as the production of ammonia and the synthesis of plastics.